
eCTD v4.0進化に関するシリーズ第3回のブログでは、新しいeCTD v4.0のモジュール1から5の主なポイントと変更点について詳しく解説します。また、eCTD v4.0提出に対応している日本、米国、EUの3地域における最新のコントロールド・ボキャブラリの違いについても解説します。さらに、改訂された規制要件に準拠した最新ツールの導入の必要性についてもお話しします。最後に、日本、米国、EUのクライアントと保健当局との協働経験に基づいた、成功するeCTD v4.0提出のための推奨ベストプラクティスを共有します。
eCTD v4.0におけるICHと地域別コントロールド・ボキャブラリの違い:日本、米国、EUの主要な相違点
eCTD v4.0では、詳細なコントロールド・ボキャブラリのリストが定められており、これらはICHおよび各地域の保健当局(PMDA)によって義務付けられています。そのため、最新のICHのコントロールド・ボキャブラリだけでなく、各地域が求める情報も理解し、円滑かつ迅速な提出を実現することが非常に重要です。ここでは、日本に対するICHおよびPMDAが義務付けるコントロールド・ボキャブラリや、日本、米国、EU間のコントロールド・ボキャブラリの地域差についてまとめています。
コントロールド・ボキャブラリ:ICH および 日本
- JP Submission Unit
- JP Category Event(イベントカテゴリ)
- JP Initial Submission Type(初回申請区分)
- JP Context of Use
- JP Submission(申請区分)
- JP Product Category(製品区分)
- JP Substance Name Type(成分名タイプ)
- JP Application(申請種別)
- JP Application Reference Reason(参照理由)
- JP Study Data Category(試験データのカテゴリ)
- JP Analysis Type(解析種別)
- JP Japanese Character Code(日本語文字コード)
- JP Terminology(Tabulation)用語(表形式)
- JP Terminology(Analysis)用語(解析用)
- ICH Context of Use
- ICH Context of Use Status
- ICH Document Type(文書種別)
- ICH Duration(期間)
- ICH Keyword Definition Type(キーワード定義タイプ)
- ICH Route of Administration for Non-Clinical Study(非臨床試験に使用される投与経路)
- ICH Species for Non-Clinical Study(非臨床試験に使用される動物種)
- ICH Type of Control(対照のタイプ)
- ICH Study Group Order(試験群の順序)
- ICH eCTD v4.0 IG Version(eCTD v4.0実装ガイドバージョン)
eCTD v4.0 統制語彙:地域による差異
| S. No | JP PMDA | US FDA | EMA |
|---|---|---|---|
| 1 | JP Submission Unit | US Application Type | eu-application-legal-basis |
| 2 | JP Category Event | US Context of Use | eu-application-reference-reason |
| 3 | JP Initial Submission Type | US CoU Keyword Definition Type | eu-application-submission-type |
| 4 | JP Context of Use | US Form Type | eu-contact-party-role |
| 5 | JP Submission | US Promotional Document Type | eu-context-of-use |
| 6 | JP Product Category | US Promotional Material Audience Type | eu-cou-keyword-definition-type |
| 7 | JP Substance Name Type | US Promotional Material Type | eu-data-classification |
| 8 | JP Application | US Submission Contact Status | eu-document-type |
| 9 | JP Application Reference Reason | US Submission Contact Type | eu-language-code |
| 10 | JP Study Data Category | US Submission Type | eu-m1-implementation-guide |
| 11 | JP Analysis Type | US Submission Unit Status | eu-manufactured-product-form |
| 12 | JP Japanese Character Code | US Submission Unit Type | eu-organisations |
| 13 | JP Terminology(Tabulation) | US Telecom Capabilities | eu-pi-document-type |
| 14 | JP Terminology(Analysis) | US Telecom Use | eu-procedure-type |
| 15 | USFDA eCTD v4.0 IG Version | eu-product-category-code | |
| 16 | eu-submission-mode | ||
| 17 | eu-submission-unit-type | ||
| 18 | eu-substances | ||
| 19 | eu-territorial-authority | ||
| 20 | eu-territorial-country-code | ||
| 21 | eu-ig-controlled-vocabularies |
なぜ eCTD v4.0 には専門的な規制申請ツールが必要なのか
eCTD v4.0 の薬事申請には、専用の高度なツールが不可欠です。さらに複雑なのは、このツールが各国・各保健当局向けの異なるモジュールを備えていなければならないという点です。これにより、各国特有の細かな違いや要件を完全に反映することが可能になります。
Freya.Fusion ― 日本PMDA向け業界初のeCTD v4.0対応ツール
eCTD v4.0対応のクラウド型規制申請支援ツールであるFreya.Fusionは、日本のPMDA向けに業界初の実稼働ツールです。以下の重要ポイントをしっかり管理できることが必須です。
eCTD v4.0仕様の複雑さ
eCTD v4.0は特有の階層構造、詳細なメタデータ要件、コントロールド・ボキャブラリの使用、複雑かつ一貫性のあるXMLバックボーンファイルの生成、そして送付書類の適切なリンク設定を必要とします。
XML生成
申請構造や文書ごとのメタデータ、相互関係を定義するXMLファイルを仕様に沿って正確に作成することが求められます。
メタデータ管理
コントロールド・ボキャブラリを含む厳格なメタデータ要件に基づき、各文書に一貫して正確にメタデータを適用・管理できる機能が必要です。
文書のリンクと構造管理
提出するすべての文書をeCTD内で正しくリンクし、PMDAの規定に適合する構成を保ちます。
検証機能
ツールはeCTD v4.0仕様およびPMDA独自要件を自動的に検証し、エラーを早期発見・修正する支援機能を備えています。
ライフサイクル管理
新規申請、修正、補足などの文書のライフサイクルを正しい形式で管理し、連続的なシーケンス作成をサポートします。
コンプライアンス保証
Freyrの専門家が監修しており、Freya.Fusionを用いることで最新の規制要件に準拠し、申請の遅延や却下リスクを最小化します。
eCTD v4.0の道をリードする ― Freyrの専門性とグローバル展開からの知見
最後に、Freyrがどのようにサービス面とテクノロジー面の両方から、世界各国でのeCTD v4.0申請をリードしているかをご紹介いたします。
当社は、日本PMDA向けeCTD v4.0モジュールをいち早く整備した企業のひとつであり、さらに米国FDA向けモジュールも2025年第4四半期までにお客様の運用に対応予定です。
本セクションでは、当社が世界各地域で得た経験や主要な学び、そしてeCTD v4.0申請を成功に導くためのベストプラクティスをご紹介します。
Freyrの実績と主な学び
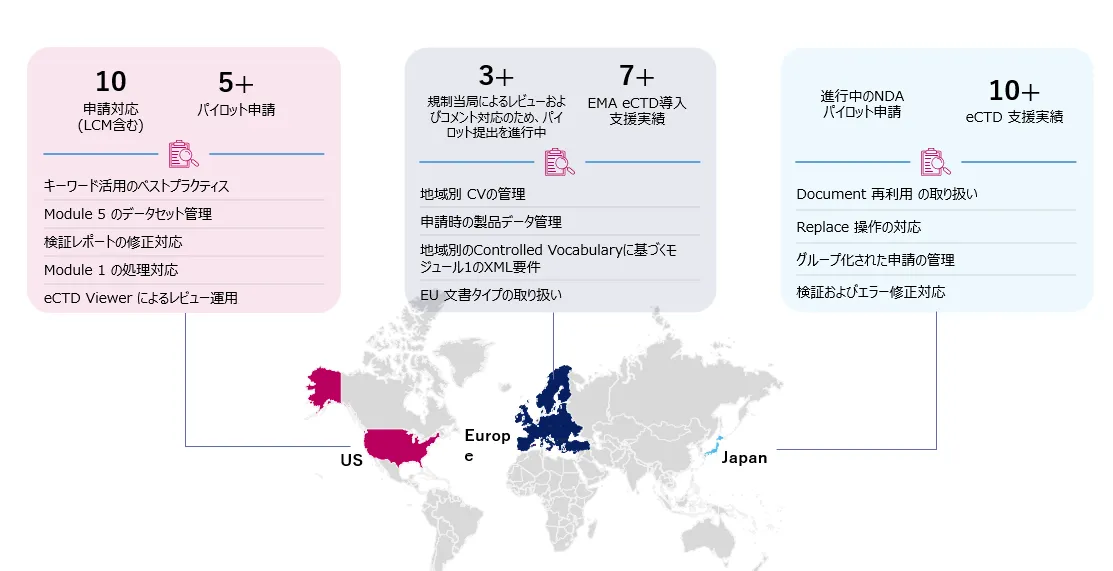
Freyr が提案するeCTD v4.0 申請のベストプラクティス
ICH および PMDAのeCTD v4.0要件 の理解
- eCTD v4.0 ガイドライン、ICH および PMDAの要件、Controlled Vocabulary、検証基準を理解する
- 最新動向を理解するため、規制専門家によるワークショップに参加する
現状のPMDA/ グローバル申請プロセスの見直し
- 現行の申請プロセスやシステムに対するギャップ分析を実施
- eCTD v4.0の新要件に 準拠していない部分を 特定する
eCTD v4.0に対応したソフトウェア・プロセス・ツールへアップグレード
- 規制当局が求めるすべての機能・構成に対応したeCTD v4.0準拠ソフトウェアを導入する
eCTD v4.0 アドバイザリー体制の 構築
- E-2-E (End-to-End) 規制サービスプロバイダーと連携する
- パートナー (例:Freyr)とプロセスおよび技術面で協働する、またはクライアントの体制と連携する
- 進化する規制要件へのコンプライアンス維持のため、業界リーダーと連携する
- グローバルおよびローカルの規制申請・運用サービスを提供するパートナー(例: Freyr )を活用する
eCTD v4.0 トレーニング
- 社内チームに向けた eCTD v4.0 トレーニングセッションを実施する
- eCTD v4.0の概要
- PMDA ガイドライン
- プロセス トレーニング
- 実践的なソフトウェア トレーニング-パイロット提出やサンプル提出
パイロット提出
- 本格導入前に、eCTD v4.0のパイロット提出または概念実証(PoC)を実施
まずはお気軽にご相談ください。貴社の業務に合わせた最適なソリューションをご提案し、スムー ズで効率的な申請を実現します。

著者について
ラガヴェンドラン・バブ(ラガヴ)氏は、グローバルな保健当局への申請業務において20年以上の経験を持つ、熟練した規制業務のリーダーです。Freyr社の規制業務ディレクターとして、規制申請戦略、eCTD/NeeS/紙媒体での申請、さらにはAIの応用や構造化コンテンツソリューションなどの革新的な規制テクノロジーにも注力しています。
業界における専門家として、eCTD v4.0、SPL、SPM、ePIなど進化する規制標準を主要市場で先導的に導入・実装した実績があります。技術チームと密接に協働し、規制ビジネスのニーズと実用的かつ拡張可能なソリューションとの整合を図る一方で、社内外のトレーニングプログラムも主導し、規制対応力の強化にも貢献しています。ラガヴ氏は、化学の学士号およびシステム&情報技術専攻の経営学修士号(MBA)を取得しています。

モヒト・バトラ博士は、医学博士であり、ライフサイエンス、デジタルヘルス、規制業務分野で12年以上にわたるグローバルな経験を有する優れた戦略コンサルタントです。現在はFreyr Solutionsにて、規制業務のグローバルビジネスおよび戦略部門の責任者(シニアディレクター)を務めており、医学的専門知識と戦略的洞察を融合させた独自の視点を提供しています。バトラ博士は、デリー大学で医学博士号(MD)を取得し、インド経営大学院(Indian School of Business)でMBAを取得しています。