
In our blog 3 of the series about eCTD v4.0 evolution, we share details on key highlights and differences in some of the modules 1 to 5 in the newer eCTD v4.0. We will also talk about the difference in the latest 4.0 Controlled Vocabularies for Japan, US and EU – the three regions which are ready for eCTD v4.0 submission, either live or pilot submission. We will also discuss the need for adopting the latest tools which comply with the updated mandates. Lastly, we will share the suggested best practices for a successful eCTD v4.0 submission based on Freyr’s experience working with clients and health authorities across Japan, US and EU.
ICH vs Regional Controlled Vocabularies in eCTD v4.0: Key Differences Across Japan, US & EU
For eCTD v4.0, there is a detailed list of controlled vocabularies (CVs) – these are mandated by both ICH and the regional health authority (PMDA). Hence, it is of utmost importance to understand not only the latest ICH mandated CVs, but also the information required by each region to ensure seamless and quick submission. Here we are listing down the CVS as mandated by ICH and PMDA for Japan and also the regional differences between controlled vocabularies for Japan, US and EU.
Controlled vocabularies – ICH and Japan
- JP Submission Unit
- JP Category Event
- JP Initial Submission Type
- JP Context of Use
- JP Submission
- JP Product Category
- JP Substance Name Type
- JP Application
- JP Application Reference Reason
- JP Study Data Category
- JP Analysis Type
- JP Japanese Character Code
- JP Terminology(Tabulation)
- JP Terminology(Analysis)
- ICH Context of Use
- ICH Context of Use Status
- ICH Document Type
- ICH Duration
- ICH Keyword Definition Type
- ICH Route of Administration for Non-Clinical Study
- ICH Species for Non-Clinical Study
- ICH Type of Control
- ICH Study Group Order
- ICH eCTD v4.0 IG Version
eCTD v4.0 Controlled Vocabularies: Regional Differences
| S. No | JP PMDA | US FDA | EMA |
|---|---|---|---|
| 1 | JP Submission Unit | US Application Type | eu-application-legal-basis |
| 2 | JP Category Event | US Context of Use | eu-application-reference-reason |
| 3 | JP Initial Submission Type | US CoU Keyword Definition Type | eu-application-submission-type |
| 4 | JP Context of Use | US Form Type | eu-contact-party-role |
| 5 | JP Submission | US Promotional Document Type | eu-context-of-use |
| 6 | JP Product Category | US Promotional Material Audience Type | eu-cou-keyword-definition-type |
| 7 | JP Substance Name Type | US Promotional Material Type | eu-data-classification |
| 8 | JP Application | US Submission Contact Status | eu-document-type |
| 9 | JP Application Reference Reason | US Submission Contact Type | eu-language-code |
| 10 | JP Study Data Category | US Submission Type | eu-m1-implementation-guide |
| 11 | JP Analysis Type | US Submission Unit Status | eu-manufactured-product-form |
| 12 | JP Japanese Character Code | US Submission Unit Type | eu-organisations |
| 13 | JP Terminology(Tabulation) | US Telecom Capabilities | eu-pi-document-type |
| 14 | JP Terminology(Analysis) | US Telecom Use | eu-procedure-type |
| 15 | USFDA eCTD v4.0 IG Version | eu-product-category-code | |
| 16 | eu-submission-mode | ||
| 17 | eu-submission-unit-type | ||
| 18 | eu-substances | ||
| 19 | eu-territorial-authority | ||
| 20 | eu-territorial-country-code | ||
| 21 | eu-ig-controlled-vocabularies |
Why eCTD v4.0 Requires Specialized Regulatory Submission Tools
Yes, there is a strong need for a tool which specializes in regulatory submission and that too in eCTD v4.0. To add on to the complexity, the tool needs to have different modules for each country / Health Authority to ensure the country specific nuances and requirements are taken into account fully.
Meet Freya.Fusion – Industry’s First eCTD v4.0 Tool for Japan PMDA
Freya.Fusion, our Cloud based regulatory tool is industry’s first tool which is live for customer use with eCTD v4.0 module for Japan. The key factors about Freya.Fusion (or any other reliable tool) that it should be able to manage the below key points:
Complexity of the eCTD v4.0 Specification
The eCTD v4.0 format involves a specific hierarchical structure, detailed metadata requirements, controlled vocabularies, a single complex XML backbone, and correct linking of all documents.
XML Generation
Generating the XML backbone file correctly according to eCTD v4.0 schema requires deep understanding by the tool and developers.
Metadata Management
eCTD v4.0 requires stringent metadata management, including controlled vocabularies. Tools must apply this metadata consistently and accurately.
Document Linking and Structure
Ensure all documents are correctly linked within the eCTD structure to comply with regulatory authority specifications.
Validation Capabilities
Validation features check submissions against eCTD v4.0 specifications and PMDA requirements to catch errors before submission.
Lifecycle Management
Manage submission lifecycles by creating sequences like new applications, amendments, and supplements in eCTD format.
Compliance
Freyr’s SMEs ensure that using Freya.Fusion, your submissions comply with the latest regulations, reducing delays and rejections.
Leading the eCTD v4.0 Journey: Freyr’s Expertise & Insights from Global Rollouts
Lastly, we would like to share with you how Freyr is leading the way for global eCTD v4.0 submission, from both a service and technology perspective. We are among the first to be ready with eCTD v4.0 module for Japan PMDA submission and are US FDA module will be live for customer use by Q4 2025.
Here we highlight our experiences, our key learnings across different global regions and our suggested best practices for a successful eCTD v4.0 submission.
Freyr Experience and Key Learnings
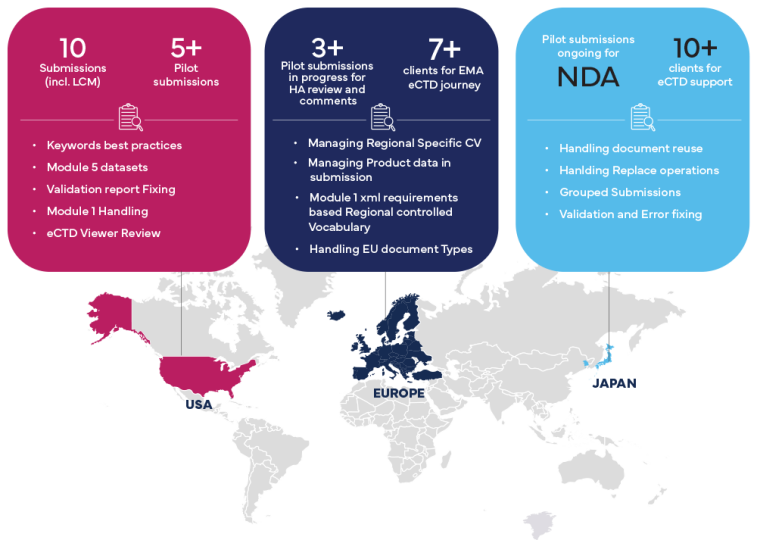
Freyr suggested Best practices for Successful eCTD v4.0 Submission
Understand ICH and PMDA 4.0 Requirements
- Be familiar with the eCTD v4.0 guidelines, ICH and PMDA 4.0 Requirements, Controlled vocabularies and Validation criteria
- Attend workshops by regulatory experts for understanding new updates
Review your existing PMDA/ Global submission processes
- Conduct detailed gap analysis on existing submission processes and systems
- Identify current process that doesn’t comply with new eCTD v4.0 requirements
Upgrade existing Software, Process and Tools for eCTD v4.0
- Deploy eCTD v4.0 compliant software supporting all functionalities and structure that meet HA mandated requirements
Collaborate for eCTD 4.0 Advisory
- Collaborate with end-to-end regulatory service providers
- Engage with partners for process, technology & compliance
- Work with industry leaders to stay compliant
eCTD 4.0 Training
- Overview of eCTD v4.0
- PMDA guidelines
- Process training
- Hands-on software training: pilot/sample submission
Pilot Submissions
- Before full scale implementation, conduct eCTD v4.0 pilot submission or Proof of concept
Curious about how we can help you achieve faster, more efficient submissions? Reach out to Freyr today!

About the Authors
Ragavendran Babu (Ragav) is a seasoned regulatory operations leader with over 20 years of experience across global health authority submissions. As Director of Regulatory Operations at Freyr, he specializes in regulatory submission strategy, eCTD/NeeS/Paper submissions, and emerging innovations in regulatory technology, including AI applications and structured content solutions.
Ragav has served as an industry subject matter expert, successfully piloting and implementing evolving regulatory standards such as eCTD v4.0, SPL, SPM, and ePI across major global markets. He works closely with technology teams to align regulatory business needs with practical, scalable solutions, while also leading internal and external training programs to build regulatory readiness.
He holds a Bachelor’s degree in Chemistry and a Master’s in Business Management (Systems & Information Technology).

Dr. Mohit Batra is a medical doctor and an accomplished strategy consultant with over 12 years of global experience in life sciences, digital health, and regulatory affairs. As Senior Director and Global Business and Strategy Head of Regulatory Operations at Freyr Solutions, he brings a unique blend of medical expertise and strategic insight. Dr. Batra holds an MD from Delhi University and an MBA from the Indian School of Business.