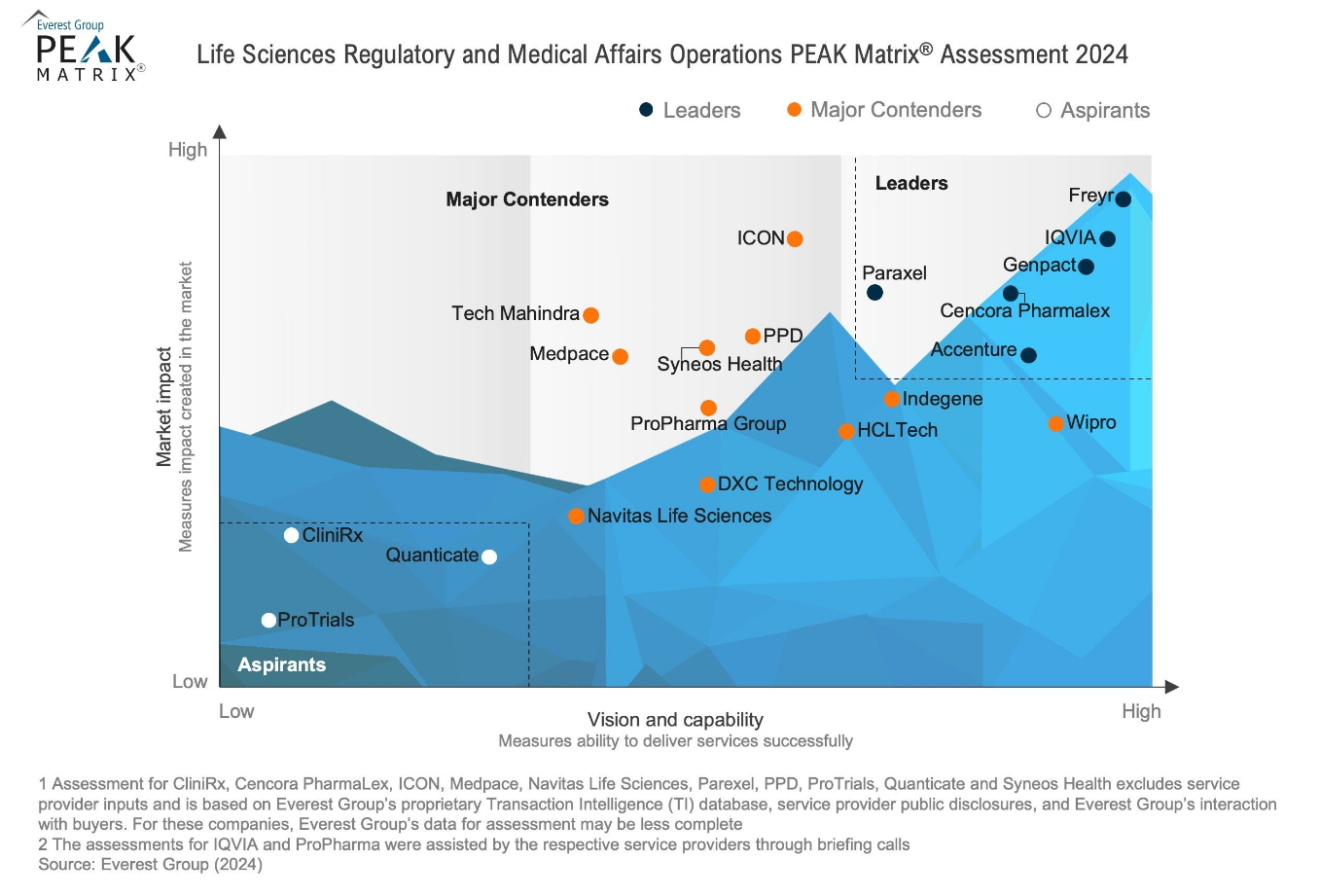
🎁 Exclusive In-Person Offer
Freyr will be providing 75 hours of FREE consultation. These can be opted for any of the below:
- 📁 eCTD v4.0 New Drug application submission to PMDA
- 🗓️ PMDA consultation services prior to NDA submission initiation
- 👨💼 Expert eCTD 4.0 Advisory and Consulting services for training your in-house regulatory teams
- 🎓 1 Full day eCTD 4.0 submission training session for your in-house teams