Japan Drug Registration and Approval - Overview
Japan is one of the largest pharma markets in the world.
As of 2024, Japan's pharmaceutical market has continued to grow steadily, with sales reaching $67.5 billion, further solidifying its position as the third-largest pharmaceutical market globally, following the United States and China.
The Pharmaceuticals and Medical Devices Agency (PMDA), operating under the Ministry of Health Labour and Welfare (MHLW), remains the regulatory authority responsible for overseeing the registration of medicinal products in Japan. Compliance with the Pharmaceuticals and Medical Devices Act (PMD Act) is mandatory for all pharmaceutical companies seeking market approval in the country.
Leveraging its in-depth knowledge of Japanese pharmaceutical product regulation, Freyr Japan continues to offer an extensive range of Regulatory services tailored to expedite the approval process for foreign drugs. From Regulatory Intelligence to comprehensive dossier preparation and submission, Freyr Japan provides essential support to ensure a seamless and successful market entry for foreign pharmaceutical companies seeking access to the Japanese market.
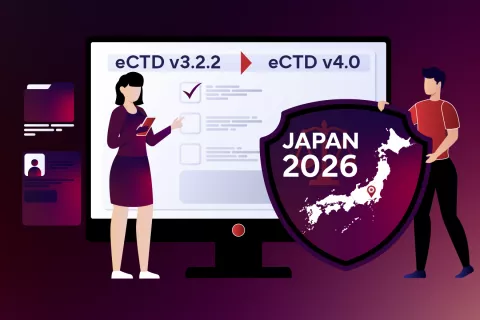
Get eCTD v4.0 Ready
Japan’s PMDA will mandate eCTD v4.0 from April 2026, bringing a smarter, metadata-driven approach to Regulatory submissions. Freyr’s local experts can help you transition smoothly from eCTD v3.2.2 to eCTD v4.0 — ensuring your medicinal product dossiers.
Stay compliant, globally aligned, and ready for faster approvals.
Drug Classification in Japan
For the registration of medicines, it is crucial to identify the product category, as the Regulatory approval process depends on that. Based on PMD-Act, drugs are classified as follows:
- Prescription Drugs
- Branded drugs
- Generic drugs
- OTC Drugs
- Quasi-drugs
At Freyr Japan, we help you identify the Japanese drug classification, the respective Regulatory approval process, and the overall Regulatory support for drugs in Japan.
Drug Registration in Japan
Under the PMD Act, the registration and approval of drugs in Japan depends on the classification of the drug product. The process for approval with the PMDA can be summarized as follows:
To import and sell drugs in the Japanese market, an entity must be registered with the MHLW. All manufacturers, foreign and domestic, are required to assign a Marketing Authorization Holder (MAH) or a Designated Marketing Authorization Holder (DMAH).
To register a pharmaceutical product, a foreign manufacturer must also be accredited by MHLW. In the PMD-Act, this process is stated as “Accreditation of Foreign Manufacturer (AFM).”
PMDA then investigates and confirms whether the manufacturing site complies with the standards of the PMDA regulations through Good Manufacturing Practice (GMP) inspections. This PMDA inspection determines the GMP conformity. Other drug quality standards include Good Quality Practice (GQP), Good Vigilance Practice (GVP), Good Clinical Practice (GCP), and Good Post-marketing Study Practice (GPSP).
An application for approval is submitted to the PMDA, which conducts a review with the help of expert committees to determine the quality, efficacy, and safety of the drugs. The documents and reports required for the application vary depending on the categories of drugs. If a foreign manufacturer of an Active Pharmaceutical Ingredient (API) does not wish to disclose its know-how in the application file, it can submit a Japanese Drug Master File (DMF) instead. In this case, it must appoint an in-country caretaker to submit the Japan DMF on its behalf. Upon reviewing the Japan DMF, the PMDA issues a DMF registration certificate.
Once the positive results of GMP conformity and Regulatory review of the application are processed by the PMDA and MHLW, the product receives approval. Approved product information is displayed on the PMDA website.
Freyr Japan supports all aspects of pharmaceutical regulation in Japan, including drug classification, selection of approval process, strategic planning, and execution of market entry.
Local Representation in Japan
Market Authorization Holder (MAH)
Foreign manufacturers should appoint a Market Authorization Holder (MAH) as a prerequisite to market drugs in Japan. However, the Pharmaceuticals and Medical Devices Act (PMD Act) allows the appointment of a Designated Market Authorization Holder (DMAH). In the MAH case, the MAH owns and controls the registration and certificate/approval of the drug. In the D-MAH case, a foreign manufacturer owns and controls the registration and certificate/approval of drugs, and the D-MAH acts as a representative in Japan. It is ideal to appoint a D-MAH rather than an MAH, as the process of changing a DMAH is easier than changing the MAH.
Accreditation of Foreign Manufacturer (AFM)
Foreign Manufacturers who plan to manufacture Medicinal products for exportation to Japan must obtain accreditation as an "Accreditation of Foreign Manufacturer ". The Minister of Health, Labor, and Welfare (MHLW) grants accreditation, while the PMDA examines the buildings and facilities of the manufacturing establishment for accreditation. The accreditation is granted for each manufacturing establishment according to the category specified by the Enforcement Regulations.
Freyr Expertise
- Regulatory Intelligence Support
- Regulatory Due Diligence Support
- Japan Drug Registration and Approval
- Submission Management to PMDA
- Data Reliability Inspection
- GMP compliance inspection
- GCTP compliance inspection
- Designated Marketing Authorization Holder (DMAH)
- Marketing Authorization Holder (MAH)
- Drug Master File (DMF) incl. In-country Care-taker (ICC) activity
- Accreditation of Foreign Manufacturing (AFM) incl. change and update activities
- Insurance Reimbursement (National Health Insurance Price Listing) Application Submission
- Labeling and Artwork Support
- Translation Services
- PMDA preliminary consultation meeting
- PMDA consultation meeting
- Orphan drug designation (ODD)
- Investigation to be applied priority review
- CTD development incl. Module 1&2 in Japanese
Validities
The Accreditation Certificate (AC) issued by the PMDA is valid for five years.
Freyr Japan provides comprehensive support for the post-approval lifecycle management of your products and overall regulatory affairs in Japan.