Our Vision

To be the essential partner for managing global Regulatory complexities and market safe and compliant products across countries.
Our Mission
To pioneer Regulatory excellence through innovative solutions and expert guidance, empowering organizations to navigate compliance complexities seamlessly and drive transformative growth.
Our Story
-
2011
Commenced operations in the United States (US) with a small set-up and a 5-member core team.
Launched Freyr EVMPD.
Roped in our first customer, a Germany-based multinational pharmaceutical company.

-
2012
Ventured into Regulatory Affairs (RA) consulting.
Assisted an American multinational corporation with RA activities.
The team grew to 7 members.

-
2013
Set up a global operational center in Hyderabad, India.
Obtained International Organization for Standardization (ISO) certifications.

-
2014
Carried out major facility expansion in India.
Expanded the Regulatory team to 50+ members.
Launched Freyr IDENTITY – a Unique Device Identification (UDI) solution.

-
2015
Expanded footprints to the United Kingdom (UK).
The team grew to 100+ members.
Initiated Regulatory services for the Medical Devices segment.
Launched a specialized Centre of Excellence (CoE) for Medical Writing.

-
2016
Set up a Regulatory hub in Germany.
Received the “India Knowledge Process Services for Life Sciences Growth Excellence Award”.
Ranked as one of the finalists for “Excellence in Pharma: Regulatory Procedures and Compliance”.
The team size expanded to a total of 150+ experts.

-
2017
Crossed the 100+ customers milestone.
Established regional offices in Mexico and the United Arab Emirates (UAE).
Launched Freyr X and partnered with in-country affiliates across the globe.
-
2018
Flourished into a new phase of growth, with 250+ global customers.
Launched the new version of Freyr SUBMIT PRO – Freyr’s in-house electronic Common Technical Document (eCTD) tool.
Pushed geographical boundaries with new office spaces in Canada, Slovenia, and Sri Lanka.

-
2019
Developed Freyr LABEL 360 – an exclusive Regulatory labeling platform.
Expanded geographically, with a new office space in the US; also established a Regulatory hub in France.
Customer base increased to 450+.
Became a strong Regulatory team with 650+ members.

-
2020
Celebrated 100+ medical device approvals.
Launched the cosmetic brand, Slova.
Developed Freyr iREADY – an exclusive ingredient database platform for Cosmetics.
With the onset of COVID-19, prioritized the well-being of employees and customers with absolute remote operations.

-
2021
Supported 2 of the 3 major COVID-19 vaccine players.
Launched Freyr Digital to provide industry-leading digital transformation solutions using Artificial Intelligence (AI), Machine Learning (ML), and Robotic Process Automation (RPA) technologies.
Celebrated 200+ medical device approvals.

-
2022
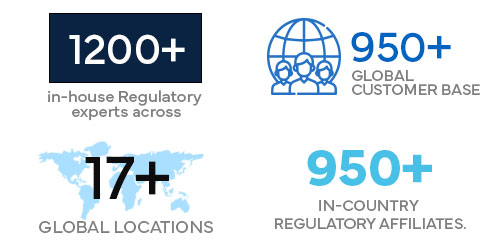
1200+ in-house Regulatory experts across 17+ global locations.
950+ global customer base.
950+ in-country Regulatory affiliates.
-
2023
Opened new offices in Colombia and Poland.
Became a strong Regulatory team with 2300+ members.
1400+ global products supported worldwide.
1450+ global customers.
Launched Freya.
Won 2+ global awards.
Collected 1000+ kgs of groceries for donations.

Global Network
The FSP Model
Freyr offers a Functional Service Provider (FSP) model, which involves outsourcing specific Regulatory functions rather than entire projects. This model provides flexibility and allows companies to maintain control over their processes while benefiting from Freyr’s expertise. Key features include:
Dedicated Teams
Regulatory experts from Freyr work as an extension of the in-house team.Scalability
Resources can be scaled up or down, based on project requirements.Cost Efficiency
Reduces costs by outsourcing only specific functions rather than entire departments.
The RAaaS Model
Freyr provides Regulatory Affairs as a Service (RAaaS), a cloud-based model where Regulatory services are delivered on demand via a subscription or pay-as-you-go basis. This model leverages advanced technology and data analytics. Key features include:
On-demand Access
Regulatory expertise and resources from Freyr are available when required, reducing the need for in-house staff.Technology Integration
This model uses Freyr’s digital platforms and tools for efficient data management and compliance tracking.Cost-effective
Under this model, you pay only for the services used, which is more economical for smaller companies or those with variable Regulatory needs.
Comprehensive Outsourcing/Full-service Model
Under this model, companies outsource the entire Regulatory function to Freyr. This includes strategy, submissions, compliance, and Post-marketing Surveillance (PMS). Key features include:
End-to-end Service
Freyr covers all aspects of Regulatory Affairs (RA), from strategy right up to execution.A Single Point of Contact
This model simplifies communication and coordination by dealing with a single service provider.Expertise and Experience
This model ensures access to Freyr’s broad range of Regulatory expertise and knowledge of different markets.
Hybrid Model
Frey’s hybrid model combines elements of both in-house and outsourced RA functions. Companies retain control over certain key Regulatory activities while outsourcing other functions to Freyr. Key features include:
Flexibility
Companies can choose which functions to keep in-house and which to outsource to Freyr.Risk Management
This model balances risk by retaining control over the critical activities.Cost Management
The hybrid model optimizes costs by outsourcing the non-core activities to Freyr.
Project-based Outsourcing
This model involves outsourcing specific Regulatory projects to Freyr rather than ongoing functions. It is ideal for companies with irregular Regulatory needs or those requiring specialized expertise for specific projects. Key features include:
Specialized Expertise
This model provides access to Freyr’s experts for complex or unique Regulatory projects.Cost Control
Fixed costs for specific projects help manage budgets effectively.Flexibility
This model provides companies with the flexibility of engaging Freyr for short-term needs.
Offshore/Nearshore Outsourcing
Under the offshore/near outsourcing model, companies outsource Regulatory functions to Freyr’s offices in different geographical locations to benefit from cost advantages and time zone differences. Key features include:
Cost Savings
This model entails lower operational costs due to geographical cost differences.24/7 Operations
Time zone differences allow for continuous workflow.Access to Global Talent
Under this model, companies can leverage local expertise from different regions within Freyr’s global network.
Co-sourcing
Under the co-sourcing model, both the company and Freyr share responsibility of Regulatory functions. This collaborative approach ensures that both parties are invested in the success of the Regulatory activities. Key features include:
Shared Responsibility
Both parties contribute their resources and expertise.Collaborative Approach
This model encourages teamwork and knowledge-sharing.Customizable Solutions
The co-sourcing model is tailored to meet specific needs and objectives of the company.
Regulatory Intelligence and Strategy Services
Freyr offers Regulatory intelligence and strategy services, partnering with companies to gain insights into Regulatory trends, policy changes, and strategic planning. Key features include:
Proactive Compliance
This model enables you to stay ahead of Regulatory changes and mitigate risks with Freyr’s insights.Strategic Planning
You can develop long-term Regulatory strategies based on the comprehensive intelligence provided by Freyr.Expert Analysis
You can conduct in-depth analysis of Regulatory landscapes and implications for the business with the help of Freyr’s experts.
Staff Augmentation
Freyr’s staff augmentation model involves providing skilled Regulatory professionals to supplement the existing in-house team of an organization. This model is useful for addressing temporary resource gaps or adding specific expertise. Key features include:
Flexible Staffing
Freyr provides Regulatory professionals on a temporary, contract, or project basis.Specialized Expertise
This model facilitates access to experienced RA professionals with specific skills.Seamless Integration
Freyr’s staff work alongside in-house teams, ensuring continuity and knowledge transfer.
Regional/Localized Delivery through Affiliate Network
Freyr's regional/localized delivery through affiliate network model involves leveraging a network of local affiliates to deliver Regulatory services tailored to specific regional requirements. This model ensures compliance with local regulations and facilitates seamless market entry. Key features include:
Localized Expertise
This model provides you with access to Freyr’s network of local affiliates who possess in-depth knowledge of regional Regulatory requirements.Customized Solutions
Through this model, Freyr helps you develop Regulatory strategies and services to meet the specific needs of different regions.Enhanced Compliance
The regional model ensures your compliance with local regulations and accelerates your market entry.Efficient Communication
Local affiliates facilitate better communication and understanding of regional Regulatory landscapes.
By leveraging the outsourcing delivery models offered by Freyr, you can enhance your Regulatory compliance, optimize costs, and focus on core business activities. Freyr’s tailored solutions provide you with the flexibility and expertise required for effectively navigating complex Regulatory landscapes.