Food & Food supplement registration in Japan - Overview
Japan's food products fall under the regulation of the Ministry of Health, Labour, and Welfare (MHLW) and Consumer Affairs Agency (CAA), governed by acts like the Food Sanitation Act and Food Labeling Act. The MHLW oversees notifications and conducts inspections for product monitoring. The MHLW's food supplement registration process involves manufacturers conducting safety and efficacy tests to ensure compliance with regulations. As a leading global Regulatory solutions provider, Freyr supports Food and dietary supplements manufacturers in Japan, by providing them end to end regulatory services as food supplement registration with the MHLW, ensuring adherence to Japan food safety regulations and standards for a seamless market entry.
Japan Food Import Regulations
The Food Sanitation Act obliges importers and manufacturers to submit import notifications to ensure the safety of imported foods and other related products. As the Food Sanitation Act states, “Those who wish to import food, food additives, apparatuses, or containers and packages for sale or use in business shall notify the MHLW on each occasion as prescribed by the Ministerial Ordinance.” Imported foods and related products should not be used for sale without proper import notifications.
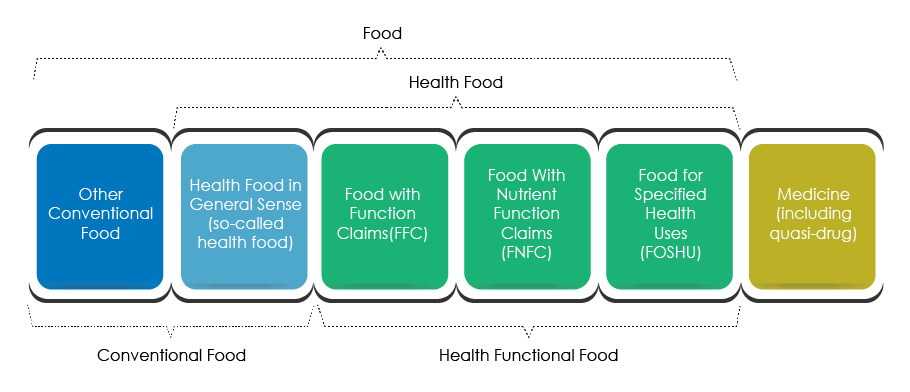
Food Product Classification
- Food with Function Claims (FFC).
- Food with Nutrient Function Claims (FNFC).
- Food for Specified Health Uses (FOSHU).
Food Product Notification
Imported foods or supplements need notification, and products labeled with specific claims must undergo either a notification or registration process.
Food Import Regulations
The Food Sanitation Act requires importers and manufacturers to notify the Ministry of Health, Labour, and Welfare about imported foods, additives, apparatuses, or containers for sale/business. Proper notifications are crucial, as per Ministerial Ordinance guidelines, to ensure safety. Sale or use of imported items without notification is strictly prohibited under the Act.
Labeling Regulations
- According to food labeling standards in Japan, labeling in the Japanese language on the primary food package is mandatory for all domestic and imported products.
- Moreover, any kind of erroneous translation of the labelings may lead to major reworking and a significant monetary loss.
Food Claims
- Foods with Nutrient Function Claims (FNCFC)
Pre-authorized nutrition claims, like "Potassium helps maintain normal blood pressure," have set conditions, including minimum levels and mandatory warnings. No notification to authorities is required for using these claims. - Foods with Function Claims (FFC):
Companies are permitted to make specific ingredient function claims for promoting and maintaining the health of individuals without any disease. These claims are the responsibility of the company and do not require pre-market approval.
Freyr Expertise
- Product Classification.
- Product Testing/Inspection.
- Ingredient Check/Pre-consultation with the MHLW.
- Label Compliance and Claims Review.
- Regulatory Intelligence (RI).
- Japan Food Container/Package Requirements.
- Dossier Compilation, Gap Analysis, and Submission/Registration.
- Food supplement registration Japan with strategic Regulatory consulting.
Freyr Advantages
- Expertise in decoding the classification of food products
- Support our clients to Comply food and supplement, packages and labels with Japanese regulations with our expertise
- An extensive partnership network across the globe
- Support in region-specific Regulatory activities
- End-to-end food regulatory consultation
- Staying up-to-date with region-specific regulations
- Strong relationship with the Health Authorities (HA)