- Commenced operations in the United States (US) with a small set-up and a 5-member core team.
- Launched Freyr EVMPD.
- Roped in our first customer, a Germany-based multinational pharmaceutical company.

- Ventured into Regulatory Affairs (RA) consulting.
- Assisted an American multinational corporation with RA activities.
- The team grew to 7 members.

- Set up a global operational center in Hyderabad, India.
- Obtained International Organization for Standardization (ISO) certifications.

- Carried out major facility expansion in India.
- Expanded the Regulatory team to 50+ members.
- Launched Freyr IDENTITY – a Unique Device Identification (UDI) solution.

- Expanded footprints to the United Kingdom (UK).
- The team grew to 100+ members.
- Initiated Regulatory services for the Medical Devices segment.
- Launched a specialized Centre of Excellence (CoE) for Medical Writing.

- Set up a Regulatory hub in Germany.
- Received the “India Knowledge Process Services for Life Sciences Growth Excellence Award”.
- Ranked as one of the finalists for “Excellence in Pharma: Regulatory Procedures and Compliance”.
- The team size expanded to a total of 150+ experts.

- Crossed the 100+ customers milestone.
- Established regional offices in Mexico and the United Arab Emirates (UAE).v
- Launched Freyr X and partnered with in-country affiliates across the globe.
- Flourished into a new phase of growth, with 250+ global customers.
- Launched the new version of Freyr SUBMIT PRO – Freyr’s in-house electronic Common Technical Document (eCTD) tool.
- Pushed geographical boundaries with new office spaces in Canada, Slovenia, and Sri Lanka.

- Developed Freyr LABEL 360 – an exclusive Regulatory labeling platform.
- Expanded geographically, with a new office space in the US; also established a Regulatory hub in France.
- Customer base increased to 450+.
- Became a strong Regulatory team with 650+ members.

- Celebrated 100+ medical device approvals.
- Launched the cosmetic brand, Slova.
- Developed Freyr iREADY – an exclusive ingredient database platform for Cosmetics.
- With the onset of COVID-19, prioritized the well-being of employees and customers with absolute remote operations.

- Supported 2 of the 3 major COVID-19 vaccine players.
- Launched Freyr Digital to provide industry-leading digital transformation solutions using Artificial Intelligence (AI), Machine Learning (ML), and Robotic Process Automation (RPA) technologies.
- Celebrated 200+ medical device approvals.

Celebrating 10th Anniversary

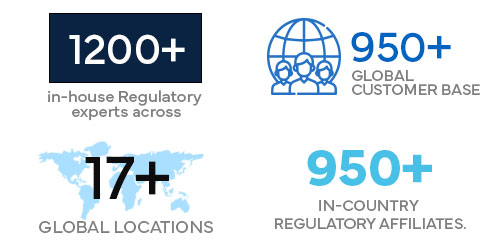
- 1200+ in-house Regulatory experts across 17+ global locations.
- 950+ global customer base.
- 950+ in-country Regulatory affiliates.
- Opened new offices in Colombia and Poland.
- Became a strong Regulatory team with 2300+ members.
- 1400+ global products supported worldwide.
- 1450+ global customers.
- Launched Freya.
- Won 2+ global awards.
- Collected 1000+ kgs of groceries for donations.
