Greetings from Freyr Japan
Freyr is a leading Regulatory solutions provider offering comprehensive services to life sciences firms across the regulatory spectrum. With a robust presence spanning over 120 countries, we facilitate successful market entry in Japan for Pharmaceutical & Lifesciences companies.
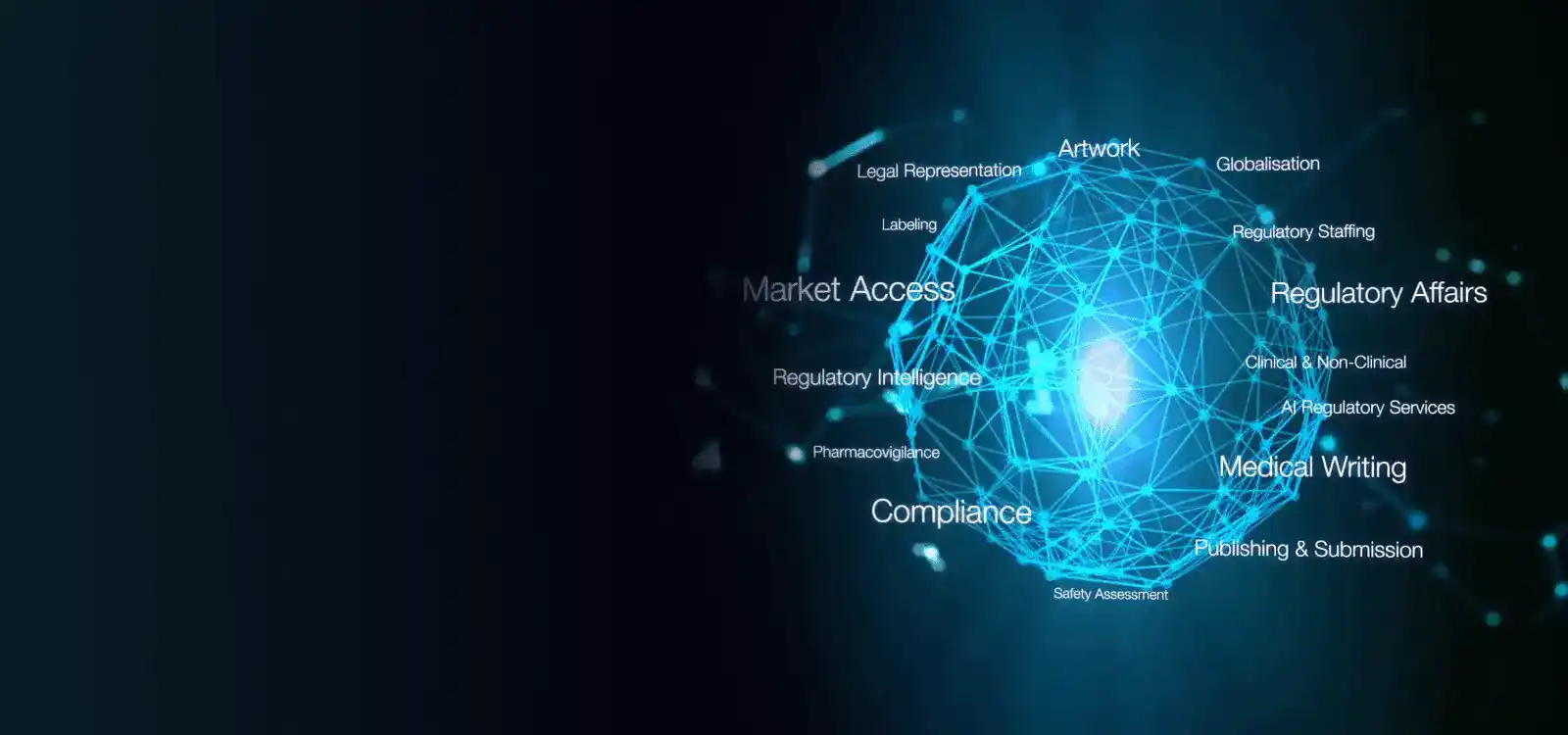
Freyr Japan is pivotal in assisting companies with product registrations under the Pharmaceuticals and Medical Devices Agency (PMDA). Our services encompass Regulatory Strategy formulation, Market Intelligence, In-country representation, Post-Market Surveillance (PMS) functions, and more. Additionally, we provide cutting-edge regulatory software solutions tailored to streamline the entire PMDA registration life cycle.
Freyr Life Sciences K.K. (the Japanese subsidiary of Freyr Inc., USA) boasts a team of seasoned experts well-versed in Japan's regulatory landscape and pharmaceutical affairs. Leveraging our international network, we offer seamless assistance for product introductions into multiple overseas markets. Our local presence ensures efficient market entry strategies tailored to your specific needs.
Global Presence in
25+ Countries2500+
Inhouse Regulatory
Experts & GrowingSupport in
120+ Countries2100+ Global
clients & growing