Launch Successfully in Japan with Strategic Regulatory & Market Entry Guidance
From product registration to Regulatory localization, Freyr powers your entry into Japan’s pharma market.
Need a Partner for Your Japan Launch?
Celebrating Customers Success
Sr. Director, Head of Regulatory OperationsIreland-based, Global Specialty Pharmaceutical Company
Medicinal Products
Publishing
UK
I am sure you have heard by now that we have received our first-ever approval from the FDA for our Brands division. This is a major milestone for us as well as for my team here in Reg Ops. I would be remiss if I didn’t point out that we would not have been able to do this without the help of your dedicated team. From the original filing, then the following year of responses, all helped get us to final approval.
Thank you Freyr team for a job well done!
Ed VenkatGlobal CMC Technical Lead
Medicinal Products
Publishing and Submission
UK
We would like to appreciate Freyr’s quick TAT to push through an urgent submission required by the FDA. Their efficiency, diligence, excellence, sense of urgency, and priority are deeply flexibility.
Please keep up the great work as we have many milestones to achieve over the next year.
Lynne McGrathRegulatory Consultant
Medicinal Products
Regulatory Affairs
USA
Please relate to the team the excellent job they have done on the IND reactivation. Specifically, the high-quality module 3, written by Freyr. It was very comprehensive, very few revisions were required, and we had no questions from Health Canada or FDA. No CMC questions: that was a first for me. So very responsive to my many requests, which I am sure can be frustrating but always helpful while producing excellent work.
Thank you for always being available and responding quickly and comprehensively to all my requests.
What a great team you have, Freyr.
Michael Bellero Sr. Director, Head of Regulatory Operations
Medicinal Products
Publishing and Submission
UK
I am sure you have heard by now that we have received our first-ever approval from the FDA for our Brands division. This is a major milestone for us as well as for my team here in Reg Ops. I would be remiss if I didn’t point out that we would not have been able to do this without the help of your dedicated team. From the original filing, then the following year of responses, all helped get us to final approval.
Thank you Freyr team for a job well done!
Vice President R&DCanada-based, Leading OTC Products Company
Medicinal Products
Regulatory Affairs
Canada
Thanks for sending the invoice. The activities and amounts generally mesh with our expectations. The provided resource is doing a great job managing our projects and keeping us informed of the status. We are very happy with her diligence, professionalism, and high level of service. For tracking these types of hourly activities in our internal records, it would be helpful if you could provide a summary of the hours by month or by country if that is possible.
System OwnerUK-based, Multinational Pharmaceutical and Biotechnology Company
Medicinal Products
Regulatory Affairs
UK
Thank You, Freyr team, for a Fantastic 2019! The OTC DOCS TD user community feels better supported despite the technical challenges and system constraints. Thank you for being the voice to represent their ways of working and helping us design a more user-friendly and effective system going forward.
Director RAUS-based, Leading Generic Pharmaceutical Company
Medicinal Products
Regulatory Affairs
USA
I just wanted to say how pleased we are with your resource's work. He has quickly come up to speed and is doing a great job.
Thank you for listening to our needs and finding such a great match.
Quality Control and Regulatory ManagerUS-based, Nutraceutical Products Manufacturing Company
Medicinal Products
Regulatory Affairs
USA
This project, eCTD DMF conversion, was against a very tight schedule and required dedicated resources. Freyr provided the dedicated resources needed to meet our immediate Regulatory filing requirements. Freyr was always on schedule/or exceeded the dates outlined in the project plan. All changes were handled within 24 hours, and notifications of changes were promptly provided. This project is not yet complete but has been very successful until this point.
Global CMC Technical LeadChina-based, Leading Innovator Pharmaceutical Company
Medicinal Products
Medical Writing
UK
We are extremely happy to inform you that the BLA was successfully submitted to the FDA. We convey our sincere gratitude to the Freyr team, who worked diligently, tirelessly, and very closely with our Bridgewater and Beijing teams over the past several months to accomplish this monumental feat on time. Freyr’s team went beyond the call of duty to make this BLA submission come true. We truly appreciate Freyr’s flexibility and eagerness to work with us to accomplish aggressive goals. Look forward to your perennial support and our continued relationship and beyond.
Your Global Partner for Local Japan Success
8+ Years of Japan Regulatory Expertise
Deep experience with PMDA processes and local compliance.
All MBL Types Secured
Demonstrating end-to-end Regulatory and operational capability.
Proven DMAH Partner
Supporting pharma and biologics clients with local representation.
Regulatory + Commercial Integration
Holistic strategies for faster, compliant market entry.
55+ Clients. One Integrated Partner.
From strategy to compliance, we cover the full Regulatory lifecycle.
Why U.S. Pharma Firms Struggle to Break into Japan

Distinct PMDA Review Structure
Requires mandatory scientific consultations, localized clinical data, and strict adherence to J-GCP, J-GMP, and GPSP-more structured than FDA protocols.
Mandatory Local MAH/DMAH
Foreign firms cannot hold product approvals directly; a Japanese MAH or DMAH is legally required to manage Regulatory, quality, and PV responsibilities.
Full Japanese Dossier Localization
Modules 1 and 2 of CTD must be submitted in Japanese; accuracy in translation is required for a high-quality dossier, which can lead to review delays.
Extended Timelines Due to Local Requirements
Bridging studies, PMDA consultations, and local quality validations often extend approval timelines beyond global launch schedules.
FDA vs. PMDA – Process Overview for Medicinal Products
| Regulatory Step | FDA (United States) | PMDA (Japan) |
|---|---|---|
| Submission Language | English | Japanese (CTD incl. Module 1&2) |
| Scientific Advice | Optional Pre-IND or Type B/C meetings | Strictly recommend formal consultations (Clinical trial protocol, Pre-NDA) |
| Local Clinical Data | Rarely required | Often required (or Japanese bridging studies) |
| Submission Pathway | IND → NDA (or BLA for biologics) | CTN → NDA (MHLW via PMDA) |
| Review Timeline (Standard NDA) | ~10 months (Priority: ~6 months) | ~12 months (Priority: ~9, SAKIGAKE: ~6) |
| Accelerated Pathways | Fast Track, Breakthrough, RMAT | Priority Review, SAKIGAKE, Conditional Approval |
| eCTD Requirements | Yes | Yes |
| Local Legal Entity Requirement | Not mandatory | MAH or DMAH is mandatory for foreign applicants |
| Pharmacovigilance Obligations | REMS if required | SMO (Safety Management Officer) of MBL Org QPPV, RMP submission, GVP/GPSP compliance |
| Post-Marketing Surveillance (PMS) | Defined case-by-case | RMP, post-marketing clinical study, re-examination, etc., are mandatory |
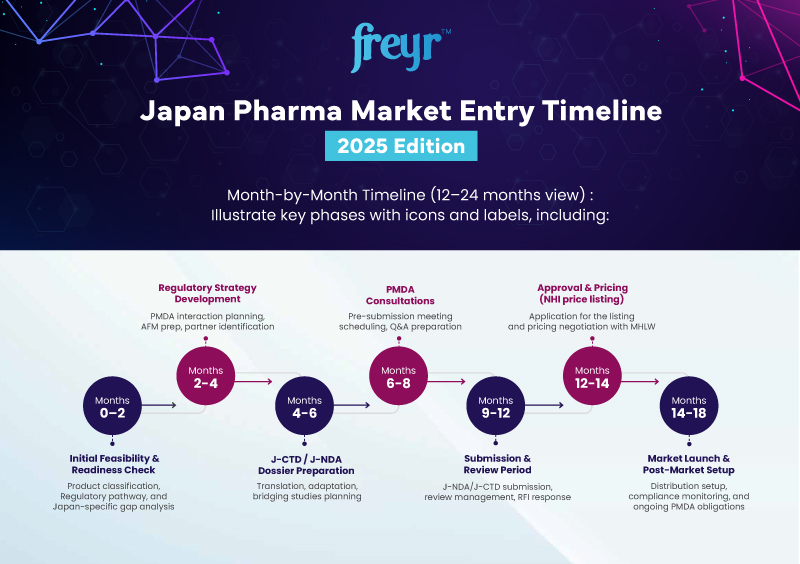
Our End-to-End Strategy: From Planning to MHLW Approval
Market Readiness Assessment
Evaluate your product’s alignment with Japan’s PMDA requirements to confirm regulatory readiness and eligibility.
Regulatory Strategy & Roadmap
Define timelines, pathways, and risk mitigation plans tailored to product class and submission type.
J-NDA / J-CTD Dossier Preparation
Compile all CTD modules or NDA documents using validated templates aligned to PMDA's expectations.
PMDA Consultation
Facilitate clinical, non-clinical, or device consultations to refine and validate your regulatory path.
Local Partner & FMA
Identify the right local agent and secure Foreign Manufacturer Accreditation (FMA) for compliance.
Launch Enablement & Post-Market
Support for NHI pricing, post-approval variations, and lifecycle compliance across Japan.
Success Stories
-
Freyr Provided Comprehensive Regulatory Support Accelerating Global Submissions and Ensuring Compliance for a Japanese Pharmaceutical and Biotechnology Company Across Multiple Markets
-
Freyr Streamlined and Standardized Submission Processes for a Japanese Drug Manufacturer's Successful Market Entry into the US Market
-
Freyr Provided Comprehensive Regulatory Support for Managing Partial Change Applications, Renewals, and Variations for a Global Healthcare Company in China and Japan
-
Freyr Provided Strategic Regulatory Partnership Achieving 100% On-Time Submissions and 50% Cost Savings with a Scalable Hybrid Staffing Model for a Japanese Midsize Global Pharma and Biotech Company
What’s New?
Frequently Asked Questions
- Japanese CTD formatting
- Translation errors
- Lack of DMAH
- Bridging study delays
- Misalignment with PMDA processes